
Exosome Diagnostics
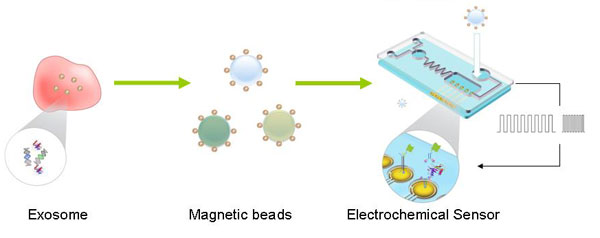
Exosomes are lipid-covered microvesicles released from various cells into bodily fluids, such as blood, urine and saliva. Exosome diagnostics based on detection of the biomarkers inside has shown great potential use in cancer therapeutics and diagnostics.The conventional way for exosome diagnostics is in a two-step mode which containing exosome extraction from the body fluids and detection of specific biomarkers with amplification associated with a variety of cancer. Direct detection of biomarkers in exosome is in vital need for both fundamental research and the clinical application.
The goal of this project is to initiate research for the direct detection of biomarkers for exosome diagnostics. The core technology to explore this project is the highly integrated electrochemical sensor, which combines the exosome extraction, biomarker release and detection. Salivary cancer biomarkers are the targeting panel in this project, however the technology is not limited to saliva exosome diagnostics. Three specific aims will address this goal: 1) Develop the technology for specific rapid exosome extraction from body fluid such as saliva via electrochemical field control and dual-marker coated nanoparticles for exosome 2) Develop the technology for specifically manipulate targeting biomarkers in exosome, including the release and the accumulation of biomarkers by the electrochemical platform. 3) Integration of the technologies on the electrochemical sensor to achieve the direct exosome biomarker detection in body fluid, which combines the exosome extraction, biomarker manipulation and highly sensitive detection process. In the preliminary study, the investigatorĄ¯s group has successfully developed the electrochemical sensor for direct detection of oral cancer salivary biomarker with high sensitivity. The proposed work will provide a general technology for direct detection of exosome biomarkers in body fluid, which will cause revolutionary improvement for exosome diagnostics.
Point-of-Care electrochemical sensor in multiplexing mode
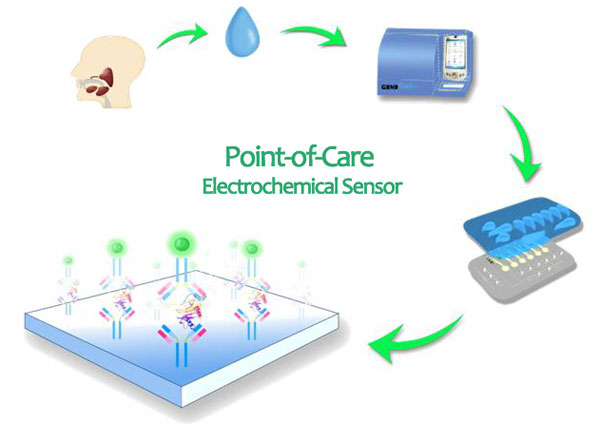
Early cancer detection is critical towards reducing mortalities and developing therapeutic strategies. Diagnostic decisions based on single biomarker are plagued with high false-positive or false-negative findings. Accurate clinical detection often requires multiplex detection of endogenous biomarkers in parallel, including proteomic and genetic biomarkers. Currently there is no technology to simultaneously detect protein and DNA/ RNA biomarkers. In addition, the technology for either protein or DNA/RNA biomarker detections require complicated sample pretreatment and signal amplification. Direct multiplex detection of biomarkers without amplification at the point-of-care has been an elusive goal for molecular diagnostics in cancer research. In the past 5 year, the principle investigator, Dr. Fang Wei, has been working in the multi-discipline team to develop a biosensor for multiplexing biomarker detection.
The goal of this project is to develop a universal strategy and tool for direct measurements of multiple nucleic acid and protein biomarkers without amplification, overcoming the major hurdles in the current methodologies for cancer detection. The core technologies, which will be used to develop the proposed biosensor, were developed by Dr. David Wong and Dr. Chih-ming Ho at UCLA under the sponsorship of two NIH U01 grants (U01DE 017790, U01DE015018). The 7 years of collective efforts result in a highly sensitive, highly specific multiplexible POC biosensor for proteomic and transcriptomic biomarkers detection in saliva [Wei, F., et al., Clinical Cancer Research, 2009. 15 p4446]. The complete set of core technologies include 1) the design of nucleic acid probe to be able to specifically amplify electrochemical signals from very few amount of target (~100 molecules) without sample extraction and amplification; 2) the technology to significantly improve the biocompatibility and probe surface density through electrode modification; 3) the technology to facilitate and enhance the process of incubation through electric waveform. The system we plan to study is the spiked saliva with nucleic acid/protein breast cancer salivary biomarkers. No Human subject will be involved. Salivary biomarker panel for the breast cancer have already been validated in the investigatorĄ¯s group.
Nano-Mosaic enhanced Multiplex Detection via Combinational Nano DNA-Dendrimer

Nano-materials have been utilized in various aspects of the biosensor by offering improved biocompatibility, additional binding sites and higher signal intensities compared to traditional materials. For the clinical diagnostics, it often requires accurate multiplexing detection of all the biomarkers in parallel. Currently the technology for simultaneously measurement of multiple protein biomarkers with high sensitivity and specificity is lacking, due to the complicated multi-protein biological system. For the complicated multiple biomarker detection, the diversity of nanomaterial provides a powerful tool for multiplex protein detection towards an elusive goal for multi-protein diagnostics. The goal of this project is to develop a general nanomaterial as Nano-Mosaic for biosensor, which contains combinational nano-materials for highly sensitive and specific multiplex detection. Combinational nano-DNA dendrimers (NDD) will be utilized to construct the nano-material. Feedback system control (FSC) is the method to optimize the composition of multiple different NDDs to achieve a global optimized sensor performance to multiple protein targets. The core technology is based on the application of randomly combinational NDD (more than four) incorporated with the conducting polymer complex as the bio-abiotic interface to detect a group of proteins. Two specific aims will address this goal with the panel of 4 pre-validated salivary proteins for the oral cancer (MRP14, CD59, Catalase, M2BP). (1) Develop the technology to construct the nano-mosaic with combinational NDD into the conducting polymer matrix. (2) Develop the method to optimize the nano-mosaic composition with combinational NDD for a group of proteins based on FSC. The optimized composition of the mixed NDD will be achieved by the FSC within limited iterations, which eliminates the huge work in multi-parameter optimization. The application of nano-mosaic with combinational NDD will overcome the major hurdles in the current methodologies for multiple biological targets detection with high sensitivity and specificity. The success of the proposed work will provide a general technology to benefit not only the nano-sensor, including the nanoprobe and nano-reporter, but the whole nanoscicence such as the nano-imaging and nanodrug. In addition, the technology implanted in the electrochemical sensor will significantly improve therapeutic applications, monitoring of therapies and assess prognosis for early disease detection.
Feedback System Control for global optimization in electrochemical sensor
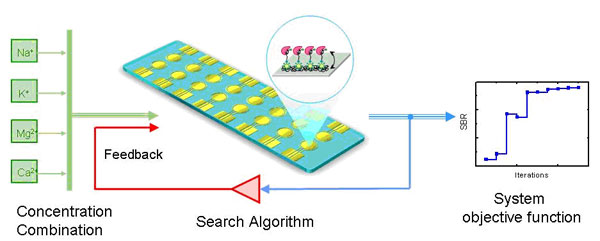
A Feedback system control (FSC) technique has been developed to rapidly identify optimal drug cocktails among 105 possible combinations for inhibiting 100% viral infection to the host cell (Sun et al. 2009; Wong et al. 2008, Ho et al. 2010, Tsutsui et al, 2011). Only about tens of feedback iterations were needed. FSC was found equally efficient in current study for determining the best combination of ions and their concentrations for rapid detection at high sensitivity. Four different ions, Na+, K+, Ca2+, Mg2+, and each with 12 different concentrations, have been used to optimize the aptamer based BoNT detection. We have found out that the convergence to optimal condition with high signal-to-background ratio (SBR) was indeed very fast. The whole process reached the optimized condition within several iterations.? The detection time is only 5 minutes and sensitivity is 40 pg/ml. Extremely time consuming titration tests of one or many chemicals commonly encounter in numerous chemistry analyses.? FSC could be an efficient approach for reducing significant time and labor efforts.
Aptamer can be used as a sensitive probe for detecting Botulinum neurotoxin (BoNT). The efficiency of binding aptamer to the target molecule depends on the formation of the proper tertiary architecture of aptamer for sensing and docking the BoNT, which is closely correlated with the types and concentrations of the ions presented in the buffer. . The sensitivity of recognizing target molecule by aptamer probe hence depends on the combinatorial effects of multiple types of ions.? Finding the optimal condition of 4 different ions with 12 concentrations, 20,736 possible combinations, by brute force is an extremely laborious and time consuming task. By applying a feedback system control (FSC) technique in this study, rapid identification of optimized ionic combinations for electrochemical aptasensor of botulium neuro toxoid type A (BoNT/A) detection has been achieved. Only about 10 iterations with about 50 tests are needed to identify the optimal ionic concentration out from the 20,736 possibilities. The most exciting finding is that very short time of detection and high sensitivity can be achieved with the optimized combinational ion buffer,. Only 5 minute, compare with hours or even days, detection time is need for aptamer based BoNT/A detection with a limit of detection of 40 pg/ml.
Bio-Abiotic Interface for clinical electrochemistry
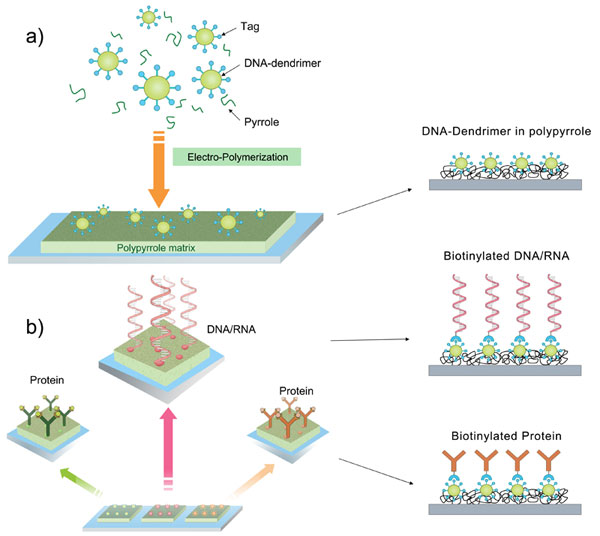
Establishing robust connection between biological and nonbiological systems will benefit understanding of biological world and in addition implement the control power into life through non-biological way. We found that combination of polymer substrate and biopolymer dopant will generate a biocompatible interface for multiple applications. Since polymer and biopolymer intrinsically share similar physical porous microstructures and surface charge properties, they are natively compatible and benefit signal transduction between biological and non-biological sides. My recent results have demonstrated the feasibility to precisely control the surface density of immobilized probe as well as to pattern the coating surface in micro-scale, by applying DNA-dendrimer nanoparticles doped into conducting polypyrrole. My future work will also be on the developments on improving sensitivity, specificity and the processing time required, which are the key requirements for biomedical analysis.